Artificial Photosynthesis: Mimicking Nature to Power the Future
Nature has been powering life on Earth for billions of years using a process called photosynthesis—the way green plants, algae, and some bacteria turn sunlight, carbon dioxide (CO₂), and water into oxygen and energy-rich sugars. It’s one of the most efficient systems for converting solar energy. But what if humans could copy this process to create clean, renewable fuels? This is the exciting idea behind artificial photosynthesis.
What Is Artificial Photosynthesis?
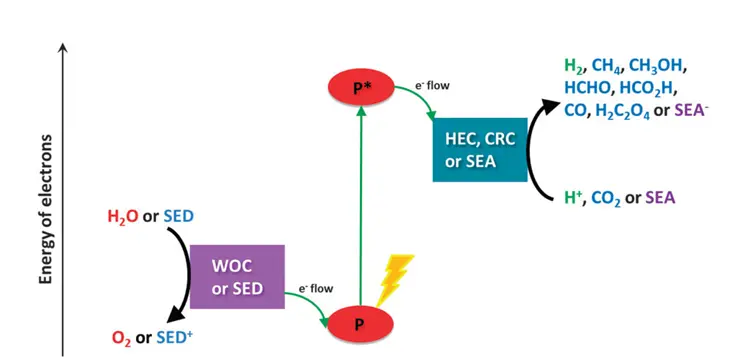
Artificial photosynthesis is a technology that imitates natural photosynthesis to produce energy in the form of fuel, usually hydrogen or carbon-based compounds like methanol. Instead of leaves and chlorophyll, scientists use photoelectrochemical cells, semiconductors, catalysts, and light-absorbing materials to capture sunlight and drive chemical reactions.
The basic inputs are:
- Sunlight – the energy source
- Water (H₂O) – for hydrogen generation
- Carbon dioxide (CO₂) – for carbon-based fuels
The outputs are:
- Oxygen (O₂) – as a by-product
- Hydrogen gas (H₂) or liquid fuels – which can be stored and used like conventional fuels

How Does It Work?
- Light Absorption
Artificial systems use materials like titanium dioxide (TiO₂) or perovskites to absorb sunlight, similar to how plants use chlorophyll.
- Water Splitting
Using catalysts, water molecules are split into hydrogen and oxygen:
- CO₂ Conversion (optional)
Some systems also capture CO₂ and turn it into carbon-based fuels, mimicking the second stage of photosynthesis (the Calvin Cycle in plants).
- Fuel Storage
The hydrogen or other fuel can be stored and used in fuel cells, engines, or even in power grids.